Mesalamine Delayed Release Tablets
Meslataj DR 400mg, 800mg, 1.2g
1. Name of the medicinal product
Mesalamine DR 400mg, 800mg, 1.2g Delayed Release Tablets
2. Qualitative and quantitative composition
400 mg, 800mg, 1.2g mesalazine per tablet.
3. Pharmaceutical form
Red-brown, oblong, modified release tablets.
4. Clinical particulars
4.1 Therapeutic indications
Ulcerative Colitis:
For the treatment of mild to moderate acute exacerbations. For the maintenance of remission.
Crohn's ileo-colitis
For the maintenance of remission.
4.2 Posology and method of administration
Swallow whole with water. Do not break, crush or chew the tablets before swallowing.
Adults:
Oral:
Acute disease: Six tablets a day in divided doses, with concomitant corticosteroid therapy where clinically indicated.
Maintenance therapy: Three to six tablets once daily or in divided doses.
ELDERLY: The normal adult dosage may be used unless renal function is impaired (see section 4.4).
CHILDREN: There is no dosage recommendation.
4.3 Contraindications4.3 Contraindications
A history of sensitivity to salicylates or renal sensitivity to sulphasalazine. Confirmed severe renal impairment (GFR less than 20 ml/min). Children under 2 years of age.
4.4 Special Warnings and precautions for use
Use in the elderly should be cautious and subject to patients having normal renal function.
Renal disorder: Mesalazine is excreted rapidly by the kidney, mainly as its metabolite, N-acetyl-5-aminosalicylic acid. In rats, large doses of mesalazine injected intravenously produce tubular and glomerular toxicity. Meslataj DR should be used with extreme caution in patients with confirmed mild to moderate renal impairment (see section 4.3). Patients on mesalazine should have renal function monitored, (with serum creatinine levels measured) prior to treatment start. Renal function should then be monitored periodically during treatment, for example every 3 months for the first year, then 6 monthly for the next 4 years and annually thereafter, based on individual patient history. Physicians should take into account risk factors such as prior and concomitant medications, duration and severity of disease and concurrent illnesses. Treatment with mesalazine should be discontinued if renal function deteriorates. If dehydration develops, normal electrolyte and fluid balance should be restored as soon as possible.
Serious blood dyscrasias have been reported very rarely with mesalazine. Haematological investigations should be performed if the patient develops unexplained bleeding, bruising, purpura, anaemia, fever or sore throat. Treatment should be stopped if there is suspicion or evidence of blood dyscrasia.
4.5 Interaction with other medicinal products and other forms of interaction
'Meslataj DR' Tablets should not be given with lactulose or similar preparations, which lower stool pH and may prevent release of mesalazine.
Concurrent use of other known nephrotoxic agents, such as NSAIDs and azathioprine, may increase the risk of renal reactions (see section 4.4)
4.6 Fertility, pregnancy and lactation
No information is available with regard to teratogenicity; however, negligible quantities of mesalazine are transferred across the placenta and are excreted in breast milk following sulphasalazine therapy. Use of 'Meslataj DR' during pregnancy should be with caution, and only if the potential benefits are greater than the possible hazards. 'Meslataj DR' should, unless essential, be avoided by nursing mothers.
4.7 Effects on ability to drive and use machines
Not applicable.
4.8 Undesirable Effects
The side effects are predominantly gastrointestinal, including nausea, diarrhoea and abdominal pain. Headache has also been reported.
There have been rare reports of leucopenia, neutropenia, agranulocytosis, aplastic anaemia and thrombocytopenia, alopecia, peripheral neuropathy, pancreatitis, abnormalities of hepatic function and hepatitis, myocarditis and pericarditis, allergic and fibrotic lung reactions, lupus erythematosus-like reactions and rash (including urticaria), drug fever, interstitial nephritis and nephrotic syndrome with oral mesalazine treatment, usually reversible on withdrawal. Renal failure has been reported. Mesalazine-induced nephrotoxicity should be suspected in patients developing renal dysfunction during treatment.
Mesalazine may very rarely be associated with an exacerbation of the symptoms of colitis, Stevens Johnson syndrome and erythema multiforme.
Other side effects observed with sulphasalazine such as depression of sperm count and function, have not been reported with 'Meslataj DR'.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
4.9 Overdose
Following tablet ingestion, gastric lavage and intravenous transfusion of electrolytes to promote diuresis. There is no specific antidote.
5. Pharmacological properties
5.1 Pharmacodynamic properties
Mesalazine is one of the two components of sulphasalazine, the other being sulphapyridine. It is the latter which is responsible for the majority of the side effects associated with sulphasalazine therapy whilst mesalazine is known to be the active moiety in the treatment of ulcerative colitis.
5.2 Pharmacokinetic properties
'Meslataj DR' Tablets contain 400 mg of available mesalazine. This is released in the terminal ileum and large bowel by the effect of pH. Above pH 7 the Eudragit S coat disintegrates and releases the active constituent. 'Meslataj DR' Tablets contain, in a single tablet, an equivalent quantity of mesalazine to that theoretically available from the complete azo-reduction of 1g of sulphasalazine.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included in other sections of the SPC.
6. Pharmaceutical particulars
6.1 List of excipients
Core:
Lactose
Sodium starch glycollate
Magnesium stearate
Talc
Povidone
Coating:
Methacrylic acid-methyl methacrylate
copolymer (1:2)
Dibutyl sebacate
Iron oxides (E172)
Macrogol 6000
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years.
6.4 Special precautions for storage
Store tablets in a dry place at a temperature not exceeding 25°C and protect from direct sunlight. Keep the bottle tightly closed
6.5 Nature and contents of container
HDPE oblong bottle with a child-resistant closure, cotton, and silica gel desiccant pouches.
Pack-sizes of 90 or 120 tablets.
6.6 Special precautions for disposal and other handling
No special requirements.
7. Manufacturer
Manufactured in India by:
TAJ PHARMACEUTICALS LTD,
220, Mahagujarat Ind. Estate, Moraiya,
Tal. Sanand , Dist. Ahmedabad,
Gujarat, INDIA.
Click here for Download pdf of patient informationClick here for Download pdf of prescribing information
Meslataj Image Gallery
Mesalamine Delayed Release Tablets Images Shown Here.
- All
- 400mg
- 800mg
- 1.2g
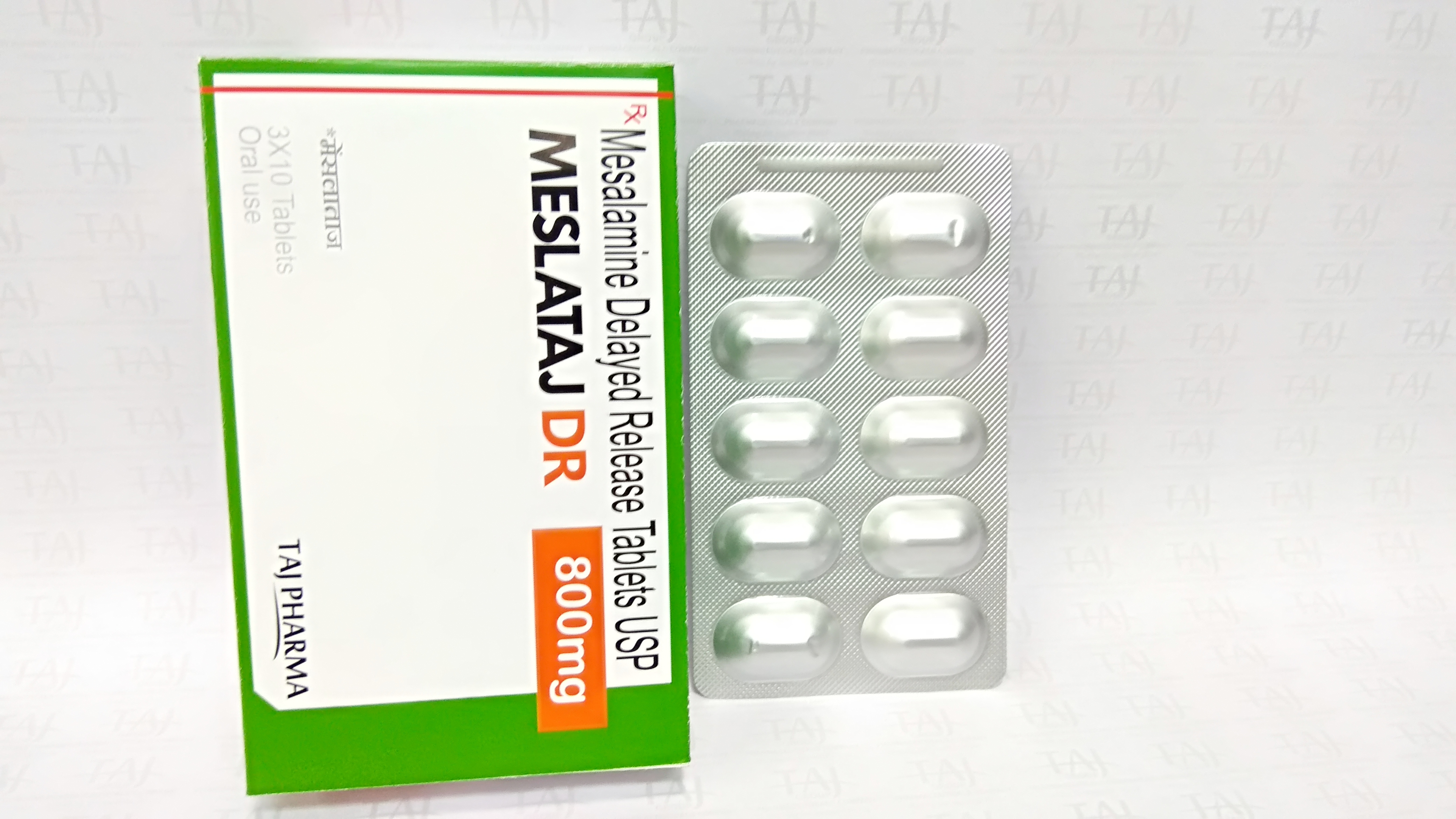
Mesalamine tablets used for
" This medication is used to treat a certain bowel disease (ulcerative colitis). It helps to reduce symptoms of ulcerative colitis such as diarrhea, rectal bleeding, and stomach pain. Mesalamine belongs to a class of drugs known as aminosalicylates. It works by decreasing swelling in the colon. "
Our partner






Contact Us
The products discussed herein may have different product labelling in different countries. Please feel free to Contact for additional information
214, Bake House, Bake House Lane,
Fort, Mumbai 400001, India.
1800-222-434
1800-222-825

Life is the underlying purpose of everything we do at Taj Pharma Group. We are committed to developing a distinguished generics pharmaceuticals business.
Taj Pharma is one of the leading generic pharmaceutical company in India. We hold top positions in different established markets and gradually building a strong presence in many emerging generics markets. We have eight manufacturing sites under flagship in India to support our market standing.
Copyright © Taj Pharmaceuticals Limited. All rights reserved. by Taj Pharma Group India.
Note: This site contains medical information that is intended for doctors or medical practitioner only and is not meant to substitute for the advice provided by a medical professional. Always consult a physician if you have health concerns. Use and access of this site is subject to the terms and conditions as set out in our Privacy Policy and Terms of Use. Copyright © Taj Pharmaceuticals Limited (India), All Rights Reserved.
